Research & Development
Our R&D approach is bold by design, built to scale, and driven by a singular mission: to discover and develop transformative therapies for patients with exceptional speed.

Our Approach to Cancer Research
Innovation that Leads with Science and Leverages Scale
At BeOne Medicines, we are purpose-built to accelerate the development and delivery of innovative medicines to address some of the greatest unmet needs in cancer.
Our team of almost 5,000 scientists, clinicians, and innovators is one of the largest oncology-dedicated research teams in the world. Across biology, chemistry, pharmacology, translational medicines and clinical science, they have a deep and nuanced understanding of cancer.
We also manage our extensive clinical research program in-house, which creates efficiencies in scale that allow for optimum decision-making and program delivery.
As we innovate with urgency, we follow a rigorous, science-forward approach to develop novel investigational medicines that:
Disrupt key drivers of cell growth and survival
Harness the body’s immune system to attack tumors
Target specific cancer biomarkers
Our Broad Clinical Program*
Targeted Innovation
Striving to Beat Cancer by Design
We focus our research on cancers with the highest global unmet need, including hematologic, breast/gynecologic, lung and gastrointestinal cancers.
To address these cancers, we have built an extensive array of novel in vitro, ex vivo and in vivo cancer models to help us:
- select scientifically sound targets
- evaluate targets and modalities that may have compelling therapeutic potential, alone or in combination
Our research is rooted in the belief that no single modality can solve the complexities of human disease. Our pipeline is built on a deliberate, modality-agnostic approach—designed to match therapeutic modality to the underlying biology of disease, the mechanistic needs of the target, and the delivery demands of each indication.
We are advancing a diverse suite of therapeutic platforms—including traditional small molecules, protein degraders, monoclonal antibodies, bi/multi-specific antibodies, antibody-drug conjugates, cytokine therapy and cell therapy—each selected and optimized for impact, precision, and scalability. This integrated, fit-for-purpose strategy enables us to pursue first- and best-in-class targets, rational combinations, and durable responses across the focused disease areas.
Discover the different modalities we’re exploring as we endeavor to create breakthrough medicines that may improve outcomes for people with cancer.
-
Small Molecules
At BeOne, we are developing traditional small molecule therapies designed to efficiently penetrate cells and reach disease–driving targets inside the body. Their low molecular weight allows for strong tissue and tumor penetration, the ability to cross the blood-brain barrier, and convenient oral dosing—offering both clinical and patient-friendly advantages.
We engineer high performing, differentiated molecules that specifically target critical oncogenic drivers, or immune cell signaling, enabling proprietary combination strategies and maximizing therapeutic impact across cancer indications.
-
Protein Degraders
At BeOne, we are pioneering a next-generation approach to targeted protein degradation through our industry-leading Chimeric Degradation Activation Compound (CDAC) platform. Unlike traditional inhibitors that block target proteins one-to-one, CDACs work catalytically – triggering the destruction of disease-driving proteins by engaging the body’s natural protein disposal system (the ubiquitin-proteasome pathway). These bifunctional molecules bind both the target protein and an E3 ligase, forming a ternary complex that drives selective protein degradation. CDACs offer several distinct advantages:
- Catalytic potency: CDACs act repeatedly and require a stable three-part complex.
- High selectivity: CDACs enable stronger, more selective targeting—even among closely related proteins.
- Increased resistance barrier: The multi-point engagement within the ternary complex makes CDACs less susceptible to resistance from on-target mutations that often undermine traditional therapies.
- Full functional knockdown: CDACs remove both enzymatic and structural (scaffolding) functions, delivering complete target inactivation.
- Access to the “undruggable”: CDACs can degrade proteins lacking conventional binding pockets.
- Reduced toxicity potential: by using E3 ligases active mainly in tumors, CDACs can be designed to minimize effects on healthy tissues.
Learn more about CDACs and how our investigational CDACs are different from other degraders.
-
Monoclonal Antibodies
Monoclonal antibodies (mAbs) are specially designed proteins that recognize and attach to specific cellular targets. Once attached, they can either help the immune system destroy the cancer or block signals that the cancer cells need to grow and survive. As cancer treatments continue to advance, we are leading efforts to develop mAbs that can be used alone or in combination with other therapies. Our goal is to create powerful treatments that directly interfere with how cancer spreads.
-
Bi/Multi-Specific Antibodies
At BeOne, we are advancing bi- and multi-specific antibody therapies designed to engage the immune system with greater precision. These engineered molecules can bind two or more targets simultaneously – such as tumor-associated antigens (TAAs) and immune cell receptors – to bring immune cells into direct contact with cancer cells and trigger targeted killing.
Our pipeline spans a broad range of immune effector cells involved in tumor surveillance, including T cell engagers (TCEs), NK cell engagers, and macrophage engagers. We are also developing next-generation TCEs with enhanced tumor specificity through conditional activation technologies and integrating co-stimulatory signals to drive deeper and more durable responses. In parallel, we are exploring rational combinations to further boost anti-tumor activity and long-term control.
-
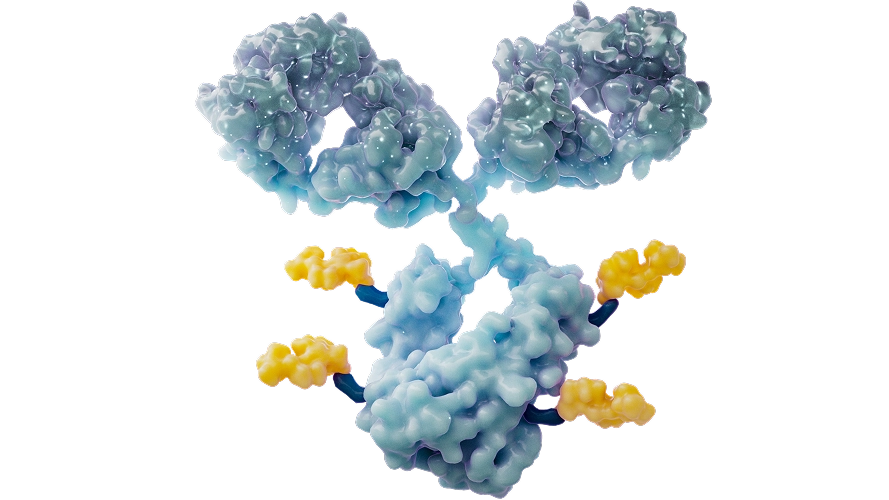
Antibody Drug Conjugates
At BeOne, we are developing next-generation antibody-drug conjugates (ADCs)—targeted therapies that deliver potent anti-cancer drugs directly to tumor cells. By combining a tumor-targeting antibody with a cytotoxic payload, ADCs are designed to maximize cancer cell killing while minimizing harm to healthy tissues.
Beyond traditional payloads like topoisomerase I inhibitors, we are advancing novel ADCs that deliver payloads targeting key oncogenic signaling pathways. This approach allows us to address well-validated targets where toxicity has historically limited clinical success. ADC technology offers a powerful solution to expand the therapeutic window and improve patient outcomes.
Our ADC pipeline focuses on dual tumor-associated antigen (TAA) targeting to widen patient coverage, overcome intra-tumor heterogeneity and deepen anti-tumor response. A dual–TAA antibody toolbox has been developed to enable seamless integration with diverse payloads, enabling rapid deployment across multiple ADC programs.
-
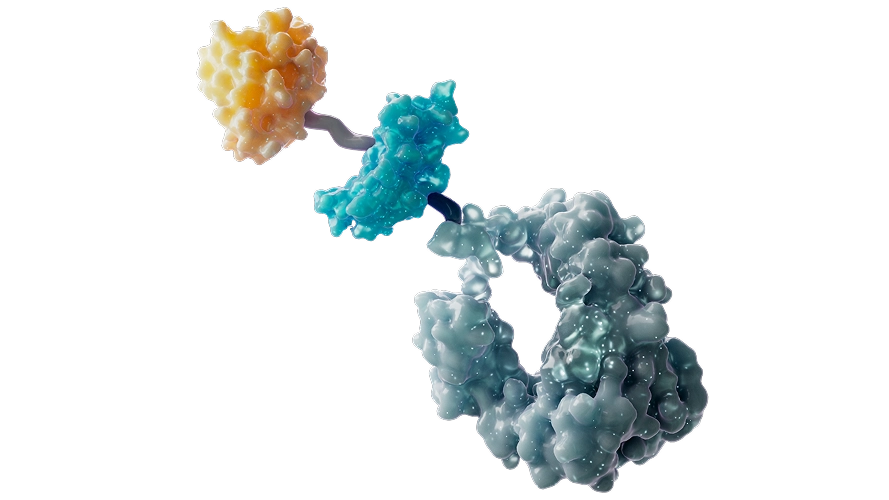
Cytokine Therapy
At BeOne, we are developing next-generation cytokine therapies designed to activate the immune system precisely at the tumor site. Cytokines are natural immune messengers, and by localizing their activity within the tumor microenvironment, we aim to boost anti-tumor responses while minimizing systemic toxicity.
-
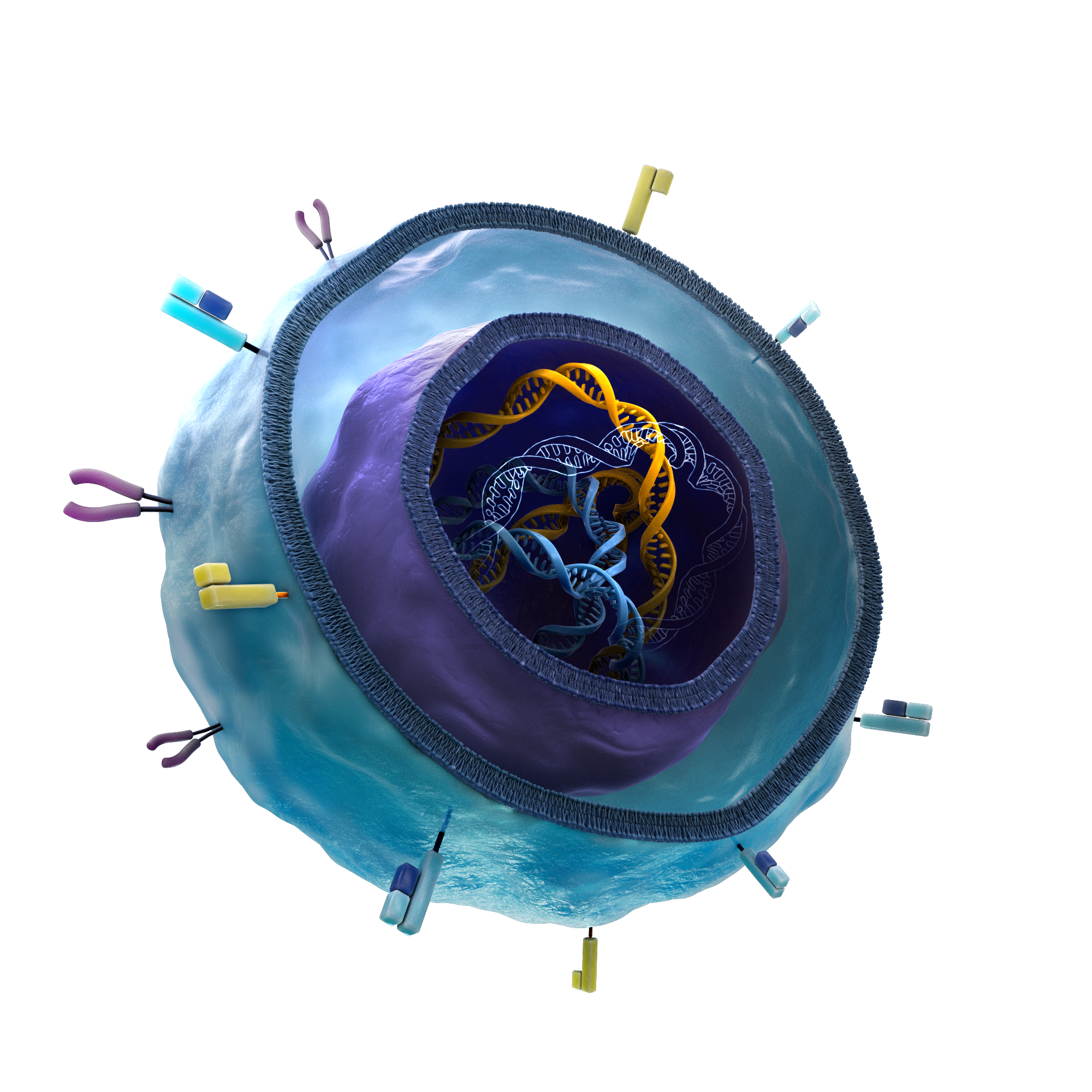
Cell Therapy
Autologous cell therapies have demonstrated strong clinical benefit, but their complex and individualized manufacturing limits broad accessibility. Early efforts in allogeneic therapies have faced challenges, particularly with cell persistence and durability.
At BeOne, we are advancing a proprietary iPSC-derived cell therapy platform designed to overcome these limitations. Leveraging the high genetic engineering capacity of iPSCs, our platform incorporates over 10 targeted edits to promote immune evasion, extend cell survival, and enhance therapeutic activity.
At the core of our innovation is a proprietary signal converter—a first-in-class technology that significantly enhances T cell killing potency and persistence. We believe this breakthrough has the potential to transform the field and unlock the full promise of off-the-shelf cell therapies.

Our Global R&D Model
Transforming Cancer Care
At BeOne, we are leveraging our strong science capabilities across diverse technology platforms to find the best interventions for each target. Dr. Lai Wang, President, Global Head of Research & Development shares how we are transforming cancer research from molecule discovery to distribution.

Innovation
See Our Pipeline
With dozens of clinical and commercial stage assets in our pipeline, we’re advancing cutting-edge science that leverages multiple technology platforms to find the best interventions for each cancer target. We’re working to deliver a precision medicine approach with the potential to improve outcomes and reduce side effects, while striving to deliver medicines sooner for people with cancer.

Driving Advances in Breast Cancer
We’re dedicated to quickly advancing differentiated treatments for breast cancers with the highest unmet need.