Guangzhou Manufacturing Site
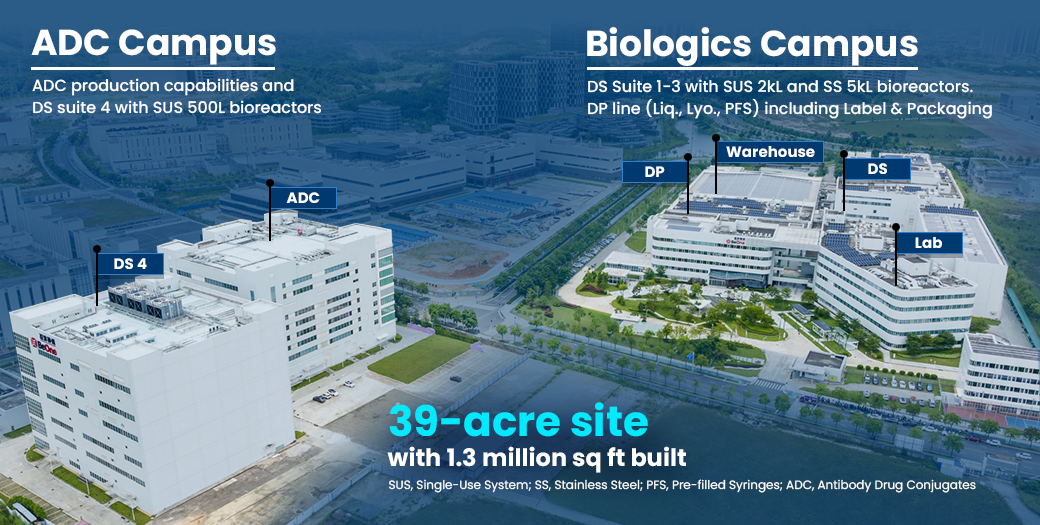
Biologics and ADC Campus
In Guangzhou, we operate a 1.3 million sq ft state-of-the-art commercial-scale facility for large molecule biologics, with a production capacity of 65,000L.
Our site also houses the first ADC R&D and production base in the Guangdong-Hong Kong-Macao Greater Bay Area, equipped with dedicated production lines, QC labs, and process development platforms to support the full lifecycle of ADC drugs.
Additionally, we own an adjacent tract of real estate for future expansion to accommodate our growing pipeline.
Drug Substance
Manufacturing Capabilities
- Total 65,000L Cell Culture Capacity
- Single-use System (2 x 500L, 12 x 2,000L)
- Stainless System (8 x 5,000L)
- Batch and Fed-batch
Drug Products
End-to-End DP Production Lines
- Liquid Vials (clinical & commercial)
- Lyophilized Vials (clinical & commercial)
- Pre-filled Syringes (clinical & commercial)
- Label and Packaging
ADCs
Conjugation and Fill Finish Production
- ADC Conjugation (up to 500L, SU & SS reactors)
- Purification with Chromatograph & UF/DF
- DP liquid and Lyophilized Vials
- Labeling & Packaging
Warehouse
Equipped with Latest Technology
- ASRS (Automatic Search & Retrieval System)
- Storage Conditions: 15 – 25°C, 2 – 8°C, -40°C, -80°C